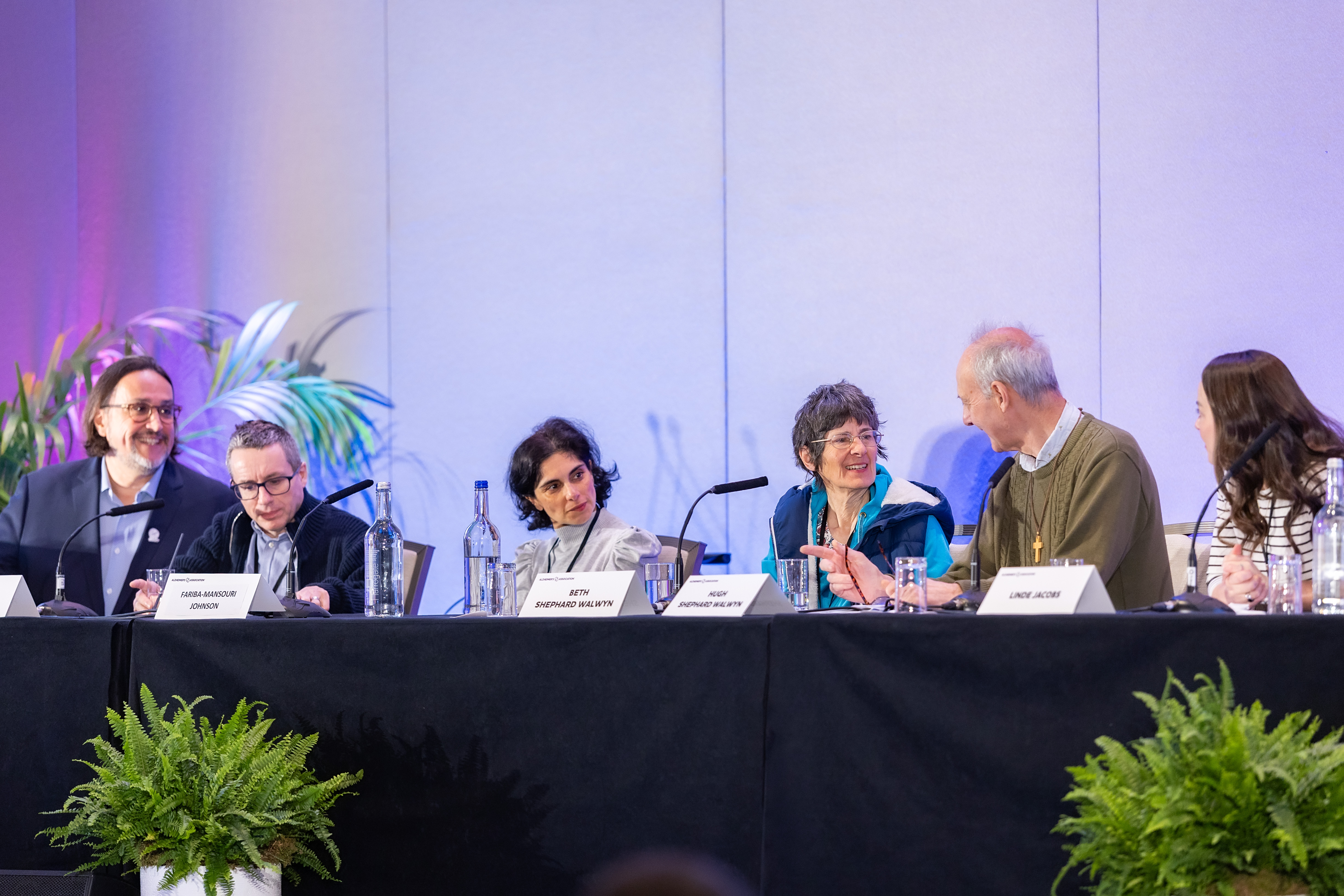Tau Global 2025 Conference Champions the Human Side of Science Amid Increasing Collaboration
May 21, 2025 Oscar Sullivan
The Tau Global 2025 Conference marked a historic convergence of three major tau research initiatives — Global Tau, EuroTau and CurePSP Neuro — bringing together an unprecedented 1240 researchers, clinicians and advocates from 64 countries, with 542 attending in-person in London. CurePSP played a central role in the conference, bridging the gap between more common diseases like Alzheimer’s and rarer ones like progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD).
In partnership with the Alzheimer’s Association and Rainwater Charitable Foundation (RCF), CurePSP supported the event for the fifth year while unveiling groundbreaking initiatives, including the PSP Trial Platform (PTP), new updates on the CurePSP Genetics Program, and future plans to study a Tau target therapy in corticobasal syndrome. The two days of scientific programming covered tau-related biology, showcased new developments in biomarkers and highlighted progress related to recent regulatory approvals of new treatments for Alzheimer’s disease. The RCF also awarded the 2025 Rainwater Prize, recognizing scientific achievement in neurodegenerative research. Kaj Blennow, M.D., Ph.D., University of Gothenburg, won the 2025 Outstanding Innovation in Neurodegenerative Research Prize. CurePSP Scientific Advisory Board member Bess Frost, Ph.D., Brown University, was awarded the 2025 Rainwater Prize for Innovative Early-Career Scientist.
The gathering underscored the growing collaborative momentum in tauopathy research, the belief that discovery depends on exchanging knowledge not just among researchers, but also with those directly affected. Kristophe Diaz, PhD, Executive Director and Chief Science Officer of CurePSP, opened day two with remarks on the tangible feeling of acceleration from the therapeutic target in Alzheimer’s that researchers are determined to use as a steppingstone for PSP and CBD treatments. Dr. Diaz stressed the urgent nature of this work, noting the millions of people who eagerly await options. As CurePSP’s Executive Director, Dr. Diaz has met countless individuals living with these conditions — an opportunity many researchers have not had. Such encounters, he says, have left an indelible mark on him. “Science needs human experiences,” he told the packed conference room before introducing the Lived Experience Panel.
The panel featured Paul, Beth and Linde, community members living with PSP, CBD and a family history of frontotemporal dementia (FTD) respectively. Beth and Paul were accompanied onstage by their spouses, who each had their arms wrapped around them as they spoke candidly about the constant uncertainty they face even after a diagnosis. They spoke with a soft clarity, recounting little changes in cognition and movement that kept occurring, as well as the piercing shock of learning that there are no treatment options available. Attendees in the crowd could be seen wiping their eyes and listening intently, signs of the lasting impression that Beth and Paul’s experiences had.
Linde then detailed her family's long history with a genetic neurodegenerative disease that claimed her grandmother, mother and all four aunts and uncles. By the time diagnoses came, years of suffering and misunderstanding had taken their toll. Genetic testing later revealed Linde and her sisters carry the same mutation, as may her young daughters.
With her scans at 36 showing early changes, Linde was confronted with knowledge that was too heavy to ignore. "Hope is not passive," she insisted, using this moment as an opportunity to change a cruel paradigm. She began donating biosamples, co-founding Cure MAPT FTD and immersing herself in research collaborations. She acknowledged the painful reality that there were no guarantees a treatment would come in time for her, but emphasized that is precisely why researchers cannot afford to slow down. "When you study my cells or scans, remember: I am still here," she urged the room. “And while I am, I will fight — not just for myself, but for every family that comes after mine.” The audience rose as one, their applause honoring the courage of all three speakers and acknowledging the collective responsibility their testimonies had instilled.
The impact of the panel lingered noticeably for the rest of the conference. Attendees throughout the day described it as “emotional,” “touching” and “a reminder of why we do this work.” Bess Frost, PhD, Director of the Center for Alzheimer’s Disease Research at Brown University and winner of the 2025 Rainwater Prize for Innovative Early-Career Scientist at the Conference, said that she had never interacted with anyone living with FTD or Alzheimer’s throughout her training, PhD and postdoc. She hopes to close the divide between scientists studying these diseases and the people living with them, as researchers can learn a lot from these exchanges.
“In the past there's been more of a movement to have patient advocates, caregivers and mutation carriers speak at conferences like this,” Dr. Frost said. “But this is the first time that I have seen an entire panel of individuals, and it's really valuable for scientists so that we can learn from each other.”
The next session echoed the panel’s call to action, as Dr. Adam Boxer, principal investigator of the landmark PSP Trial Platform at the University of California, San Francisco (UCSF), announced the selection of the first two promising drug candidates — Axon Neuroscience's AADvac1 and Alzprotect's AZP2006 — to be evaluated in the NIH-funded initiative. The trial platform model has been an exciting development that now comes to fruition for PSP, as it allows multiple therapies to be evaluated simultaneously against a shared control group. The need for only one placebo to multiple active treatments means that more patients will be able to access prospective therapeutics.
“We've been working toward this phase of development for AZP2006 for years,” said Philippe Verwaerde of Alzprotect. “The platform trial represents our best opportunity to demonstrate clinical proof of concept while accelerating access for patients who currently have no options.” Michal Fresser of Axon Neuroscience emphasized how the collaborative structure benefits all stakeholders: "The ability to work across 50 sites with leading researchers while sharing data and infrastructure represents exactly the kind of paradigm shift our field needs." He believes that the launch of PTP marks an era of transparency between public and private investors.
“It's important to run trials where data can be shared among the industry because even if we are not successful, we can avoid the mistakes that were done previously,” Fresser said. “Companies are not sharing the data that can be leveraged for different companies, but in the end, we all have the same goal: we want to find a cure for PSP patients.”
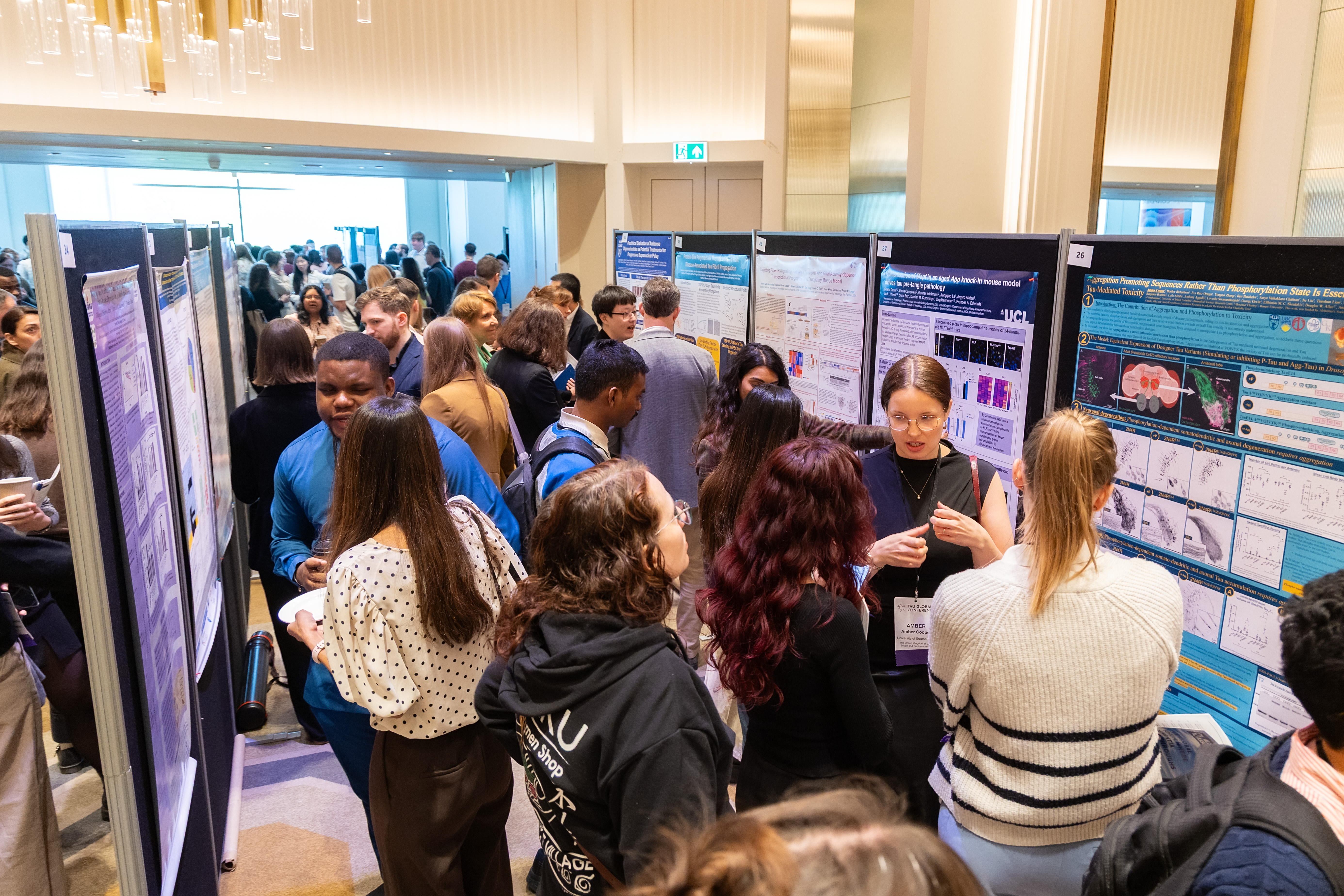
In the poster session, where emerging researchers showcase their studies to attendees, Chinyere Obasi, a Clinical Research Coordinator at Massachusetts General Hospital (MGH), shared the latest from his work on the CurePSP Genetics Program. Anne-Marie Wills MD MPH, a neurologist at MGH and Director of the CurePSP Center of Care at MGH, is the Principal Investigator of the program, a nationwide initiative that seeks to increase our understanding of these diseases through the participation of CurePSP’s community members throughout the United States. Less than a year in, Obasi says that they are already finding a slightly increased amount of familial PSP and other atypical parkinsonisms than they originally thought.
“We've been able to build some really interesting pedigrees of participants to visualize how the disease passes through families and affects family members that will be very valuable to researchers,” Obasi said.
He has particularly enjoyed the enthusiasm of participants thus far, insightful people from all backgrounds who are eager to help fill in our pictures of these diseases. Through these interactions and exchanges, the Conference cemented the necessary role of the patient perspective, a human element that will accelerate findings.
“Everything in this study has been so family-based and so personal, it’s been truly wonderful,” Obasi said. “These perspectives are not extraneous details, but deeply important to how we engage with those in our studies.”
Experts will have a year to digest the latest breakthroughs before reconvening next May for the Tau Global 2026 Conference in Washington, D.C. Later that year, the research community will return to London in the fall for Neuro2026: The PSP and CBD International Research Symposium (date to be announced later this year). Organized by CurePSP and PSPA, the organization hopes to present new updates on first results from the PTP’s inaugural treatments, while continuing to bridge the gap between the laboratory and homes of families, building on the vital patient perspective.
Read the full press release on the innovative PTP here.
Learn more about the Genetics Program here.
Check out the full photo album here.
Join our email list
Get the latest news and resources
directly to your inbox.
Get the latest news and resources directly to your inbox.
Sign Up